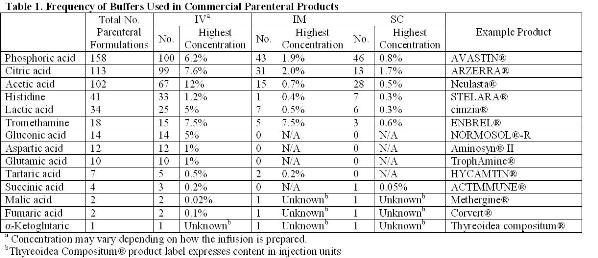
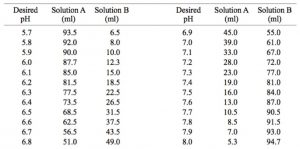
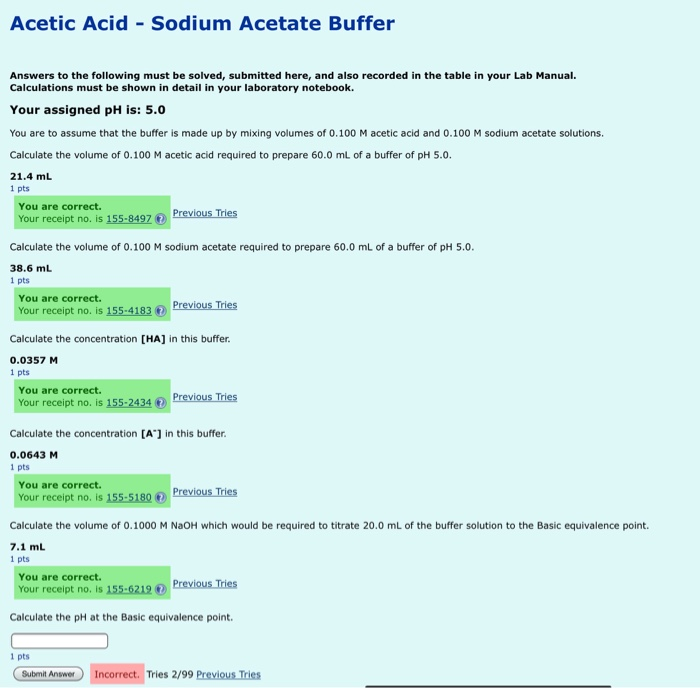
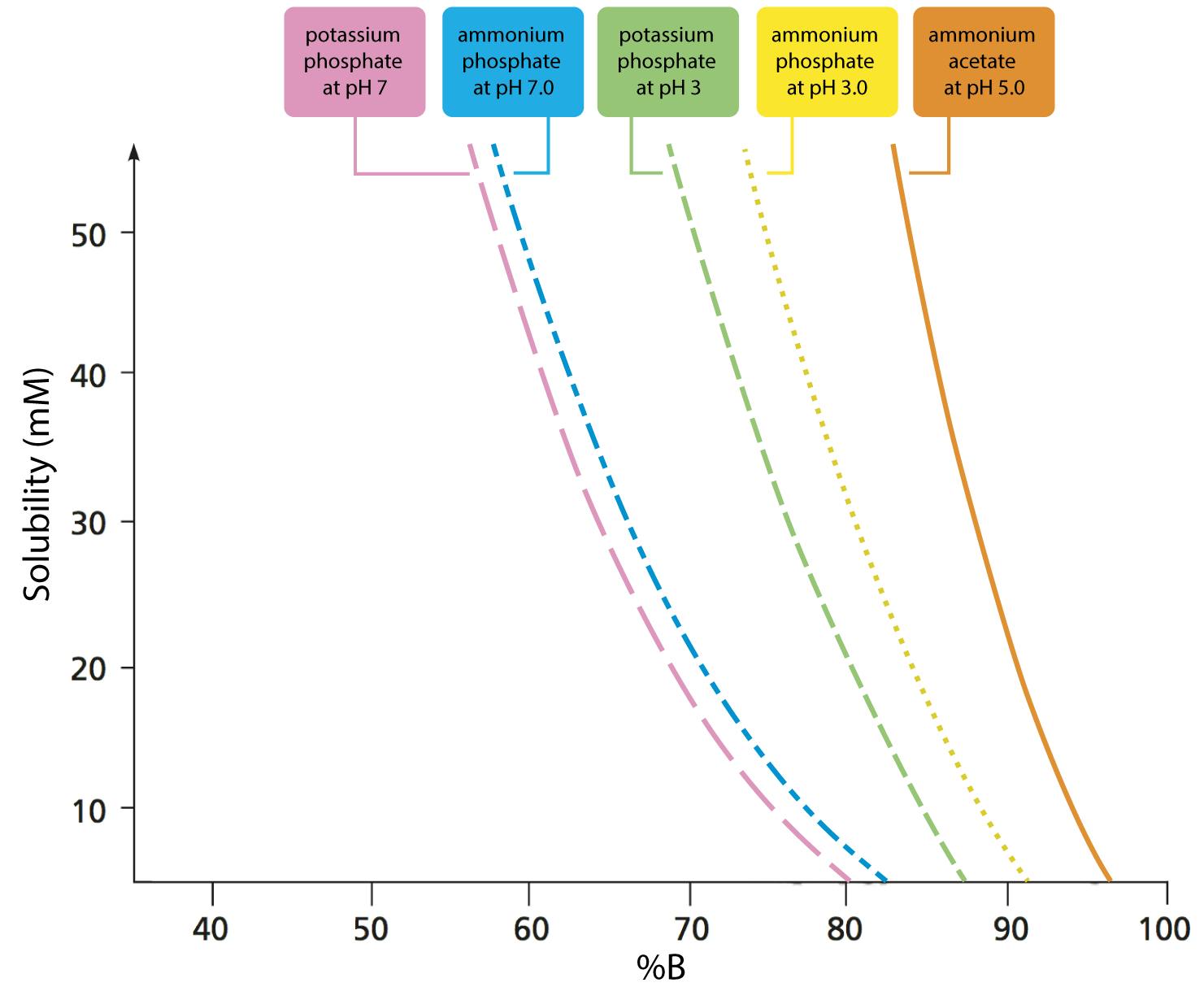
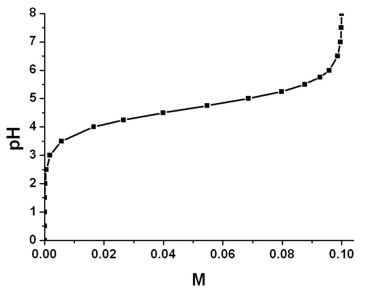
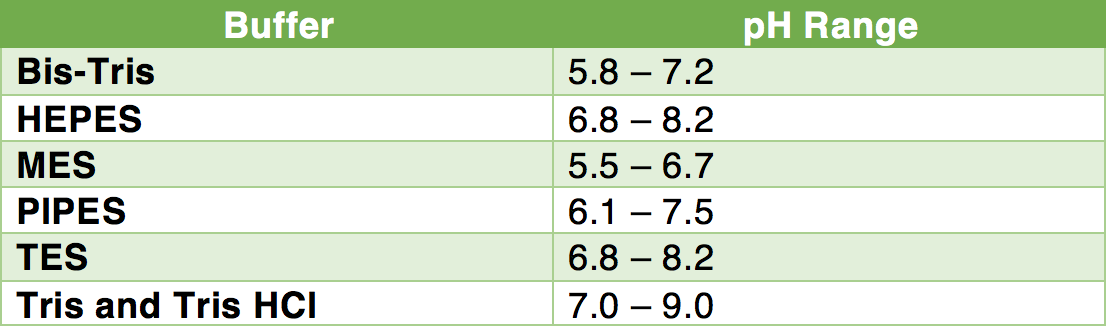
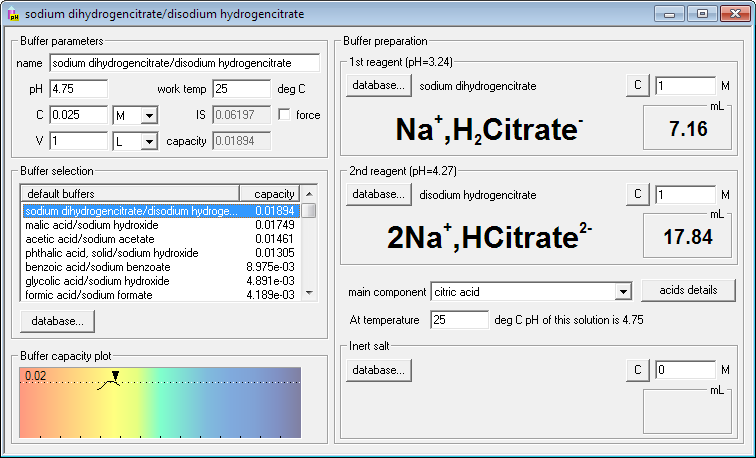
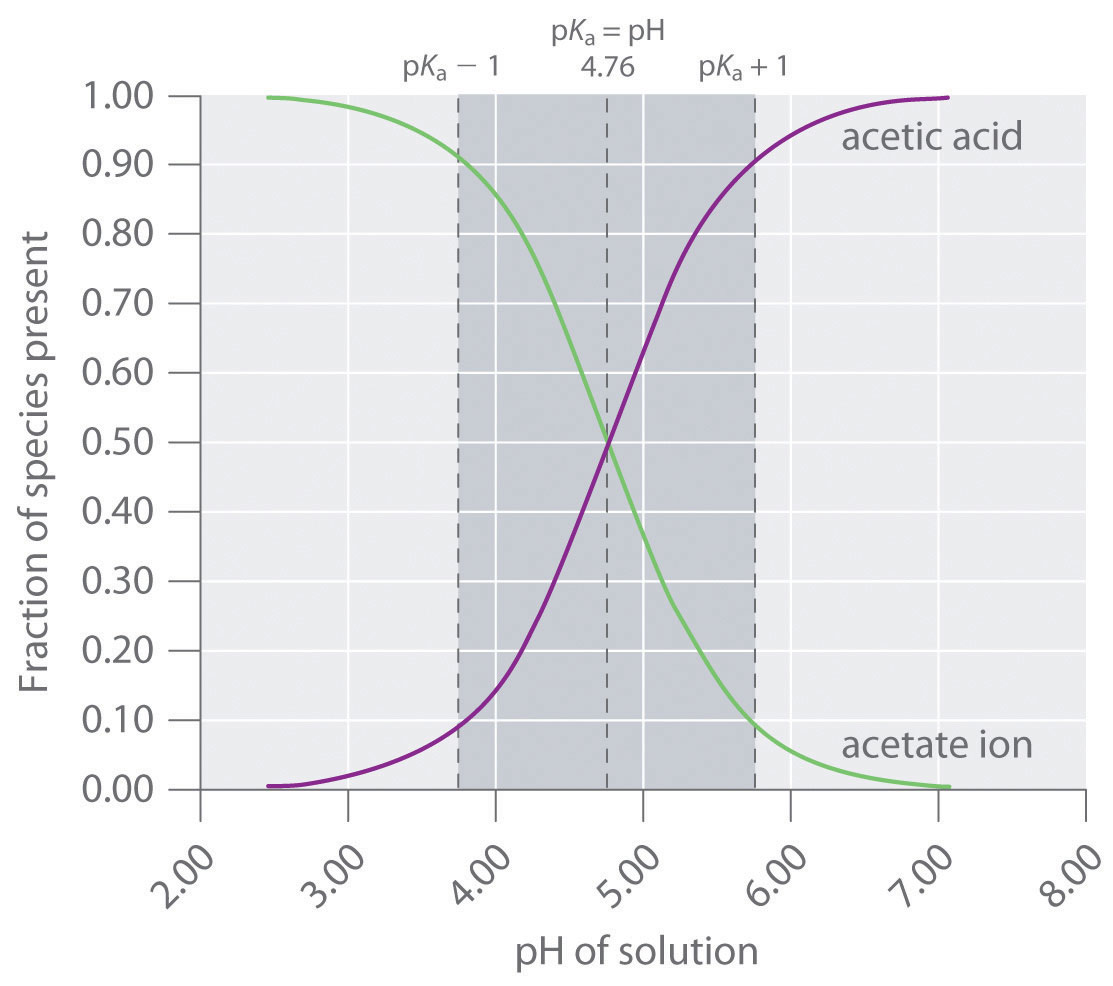
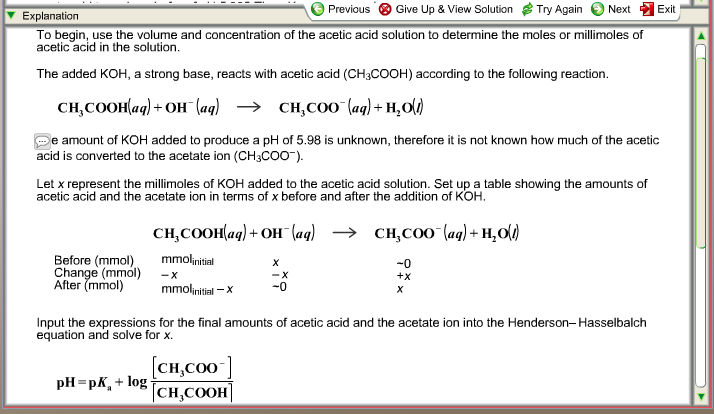
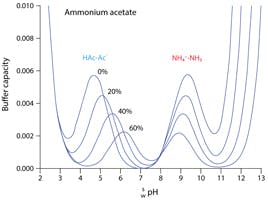
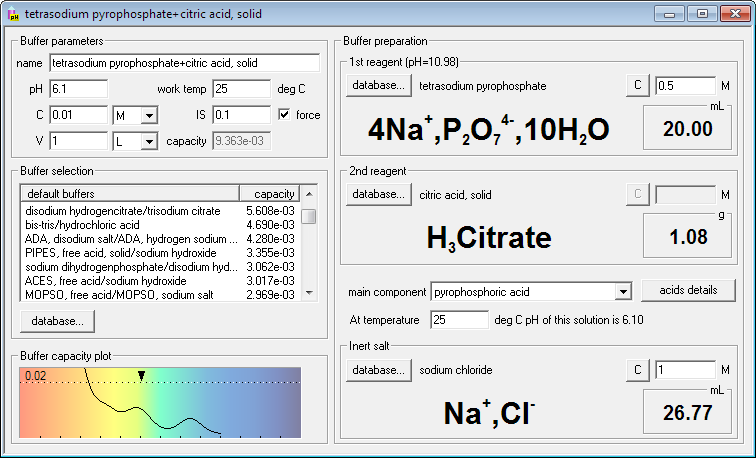
Solved] 1. Explain what is in a buffer. Discuss the function of a buffer. How will pH change when small amounts of acids or bases are added to the b... | Course Hero

Acid-Base Buffers Equation & Examples | How to Calculate pH of a Buffer - Video & Lesson Transcript | Study.com
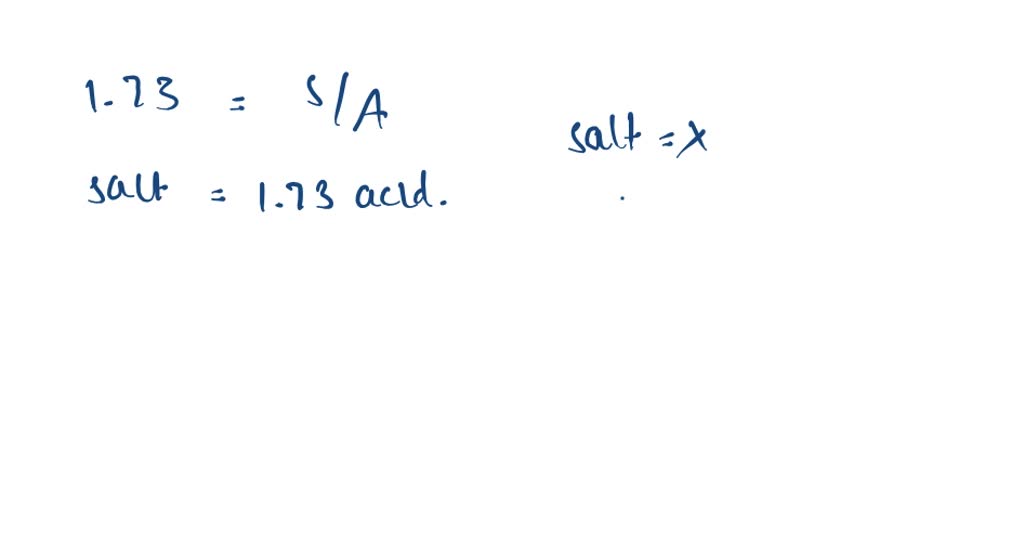
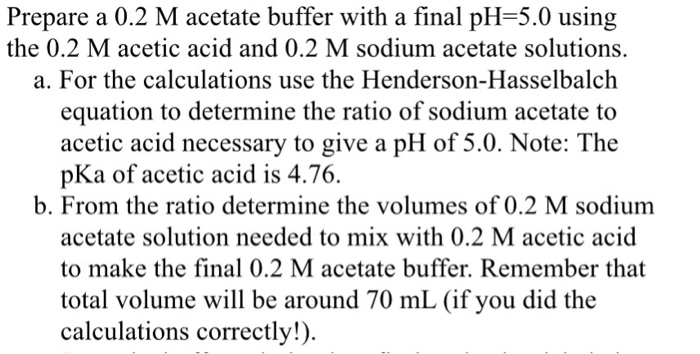
SOLVED:Preparation of an Acetate Buffer Calculate the cocentrations of acetic acid \left(\mathrm{p} K_{\mathrm{a}}=\right. 4.76 ) and sodium acetate necessary to prepare a 0.2 \mathrm{M} buffer solution at \mathrm{pH} 5.0 .
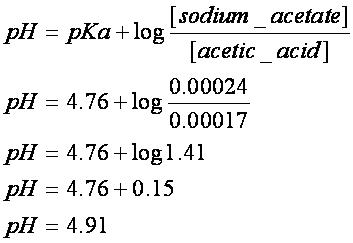
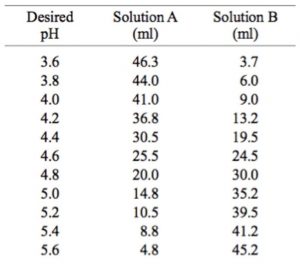
pH calculations and more in fundamentals of pharmaceutics. : Calculate pH of 100 ml buffer solution containing 0.1 g acetic acid and 0.2 g sodium actetate.